Alert, Alert, Alert: NeurAxis, Inc. (NYSE American:NRXS) volume has been increasing over the last week with significant increases in PPS, keep reading to find out why.

| We are finished with our research report on a possible rocket ship based on 4 key catalysts which may turn this NYSE stock into of of the best of the year. Take a moment and pull up NeurAxis, Inc. (NYSE American:NRXS) and start your research. In the meantime, continue reading the 4 catalyst that could spark yet another bullish move for NRXS. |
| Let’s go over some of the basics before we get into some very exciting news that all traders should be very interested in.Corporate Name: NeurAxis, Inc.Ticker: (NYSE American:NRXS) Stock Exchange: NYSE(NYSE American:NRXS) Summary:NeurAxis, Inc. Is a biotechnology company that specializes in developing innovative therapies for neurological disorders. The company has a strong pipeline of products that target various diseases, such as Alzheimer’s, Parkinson’s, epilepsy, and stroke. Here are the top four reasons why NeurAxis, Inc. is a possible good investment: |
| Why do we love this breakout alert? 1. Competitive Edge 2. Growing Customer Base 3. Explosive News 4. Bullish Chart Let’s go over the #1 reason to turn your research to NRXS. |
| 1. Competitive Edge NeurAxis, Inc. is a biotechnology company that specializes in developing innovative treatments for children’s irritable bowel syndrome (IBS). IBS is a common disorder that affects up to 20% of children and adolescents, causing abdominal pain, bloating, diarrhea, and constipation. IBS can have a significant impact on the quality of life, school performance, and social functioning of children and their families. NeurAxis, Inc. has a competitive edge in the children’s IBS market for several reasons. First, we have a proprietary platform that uses neural stem cells to modulate the gut-brain axis, which is the communication network between the digestive system and the central nervous system. By targeting the root cause of IBS symptoms, our platform has the potential to offer a safe, effective, and long-lasting solution for children with IBS. Second, we have a strong pipeline of products that are in various stages of clinical development. Our lead product, NX-001, is a neural stem cell therapy that has shown promising results in phase 2 trials for reducing pain and improving bowel function in children with IBS. We are currently preparing for phase 3 trials and expect to submit a biologics license application to the FDA by 2025. Our other products, NX-002 and NX-003, are oral formulations of neural stem cell-derived factors that have shown efficacy in preclinical models for modulating inflammation and motility in the gut. Third, we have a experienced and dedicated team of scientists, clinicians, and business professionals who are passionate about improving the lives of children with IBS. We have established collaborations with leading academic institutions, pediatric gastroenterology centers, and patient advocacy groups to advance our research and development efforts. We have also secured funding from reputable investors who share our vision and support our growth. In summary, NeurAxis, Inc. is a leader in the field of children’s IBS and has a unique value proposition that sets us apart from our competitors. We believe that our innovative platform, robust pipeline, and talented team will enable us to deliver breakthrough therapies for children with IBS and create value for our investors. Citation: https://ir.neuraxis.com/ 2. Growing Customer Base NeurAxis, Inc. is a leading company in the field of neuromodulation, offering innovative solutions for chronic pain management. One of their flagship products is the IB-stim, a small and discreet device that delivers electrical impulses to the cranial nerves, reducing the perception of pain and improving the quality of life of patients. The IB-stim has been clinically proven to be effective for various conditions, such as irritable bowel syndrome, migraine, fibromyalgia, and neuropathic pain. It is also easy to use, as it can be controlled by a smartphone app that allows users to customize their treatment settings and monitor their progress. NeurAxis, Inc. is committed to growing its customer base and expanding its market reach. The company has recently partnered with several health insurance providers and medical centers to make the IB-stim more accessible and affordable for patients. The company also invests in research and development to improve the performance and functionality of the IB-stim and to explore new applications for neuromodulation. NeurAxis, Inc. believes that neuromodulation is the future of pain management, and that the IB-stim is a game-changer for millions of people who suffer from chronic pain. By providing a safe, effective, and convenient alternative to opioids and other medications, the IB-stim can help patients regain control of their lives and enjoy a better well-being. Citation: https://ir.neuraxis.com/ 3. News – September 12th, 2023NeurAxis Inc (NYSE: NRXS) announced the publication of a Prospective study evaluating the efficacy of IB-Stim in children featured in the September 2023 Frontiers in Pain Research. The publication investigated changes in gastrointestinal symptoms and quality of life in 31 adolescent patients aged 11 – 18 years with functional abdominal pain disorders (FAPDs) before and after treatment with IB-Stim. Following IB-Stim treatment, the study noted that: Patients reported significant reductions in abdominal pain, nausea, disability, and anxiety from baseline to week 4 (p < 0.05). Parent assessments reported significant improvement in the child’s quality of life based on physical function, psychosocial function, and generic core scale scores (p < 0.05). Parents also reported reduced abdominal pain, functional disability, and somatization in their children. The global health scores also significantly improved based on patient and parent reports (p < 0.05). IB-Stim therapy is its proprietary Percutaneous Electrical Nerve Field Stimulation technology. IB-Stim is FDA-cleared for functional abdominal pain associated with irritable bowel syndrome (IBS) in adolescents 11-18 years. Press Release 5. The Chart – Take a look at this beast below, this may be the start of something truly monumental. |
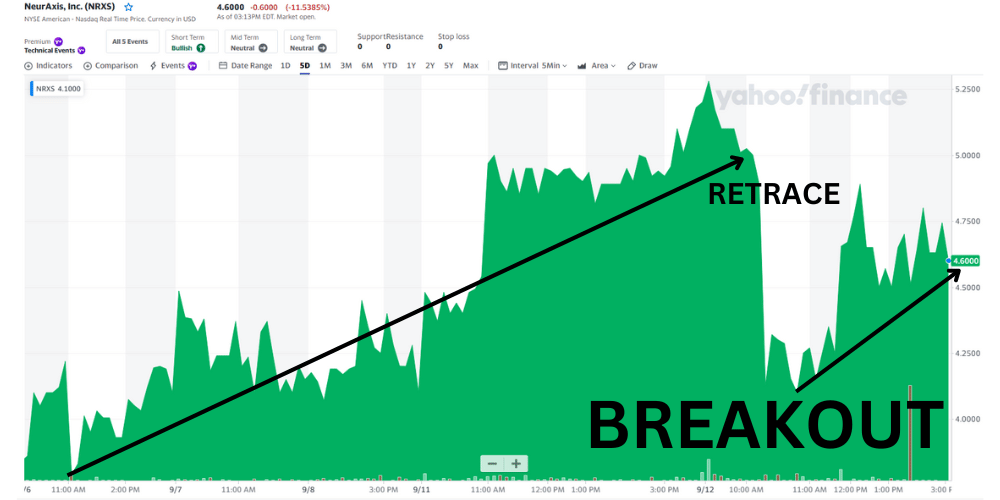
It is clear volume is increasing and there was a pullback from $5.30 to $4.10 creating a new bottom and then proceeded to breakout.
It appears that NRXS is possibly getting ready for another move from the $4.60 range which should try to test it’s latest of $5.28. Tomorrow could be a very fun day if it beats the 5 day high of $5.28, so buckle up traders!
Condensed Disclaimer
Disclaimer
The site is used for information purposes and not intended for trading purposes.
This Article contains forward-looking statements that involve risks and uncertainties, which may cause actual results to differ materially from the statements made. When used in this document, the words “may”, “would”, “could”, “will”, “intend”, “plan”, “anticipate”, “believe”, “estimate”, “expect” and similar expressions are intended to identify forward-looking statements. Should one or more of these risks and uncertainties occur or should assumptions underlying the forward-looking statements prove incorrect, actual results may vary materially from those described herein as intended, planned, anticipated, or expected. This article is not intended to be a solicitation to buy or sell securities and readers are cautioned to consult their own financial advisors before doing so.
Small Cap Exclusive owned by King Tide Media has been hired by to disseminate public information on PTN starting on 9/12/23 and ending on 9/13/23. We have been compensated up to $10,000 USD to profile NRXS. We do not own any shares in NRXS.