Good Morning Traders,
Cybin CYBN issued blockbuster news this morning that the FDA has cleared their proprietary molecule for the phase 2a study. Take a look at the news below and get ready for a big day.
Cybin Inc. (NYSE American: CYBN) today announced that the U.S. Food and Drug Administration (“FDA”) has cleared its investigational new drug (“IND”) application for CYB004, its proprietary deuterated dimethyltryptamine (“DMT”) molecule in development for the treatment of Generalized Anxiety Disorder (“GAD”). This clearance allows the Company to proceed with its plans to initiate a Phase 2a study of CYB004 in Q1 2024. The Phase 2a study will be a randomized, double-blind, active-controlled trial to assess the preliminary clinical efficacy, safety, tolerability, PK and PD of CYB004 in participants with GAD. This trial will be conducted at study sites in the United States. Press Release
If you are looking for a possible blockbuster stock in the biotech sector, turn your eyes to Cybin (NASDAQ:CYBN).
It has been a while for SCE to find a stock that we believe has what is takes to keep our streak alive and we think we may have found it! REMINDER, We are going for 8 in a row and we are feeling pretty good about CYBN. Why are we confident in Cybin (NASDAQ:CYBN)? Take a look at these price targets below by these respected Wall Street analysts.

<<Keep reading for the link to the price targets and a full analysis.>>
Now keep in mind it is trading at $.36!
Let me repeat, it is trading at $.36 and the chart looks ready for a breakout.
Cybin is one of the leading players in the psychedelic space, with a diversified pipeline of deuterated psilocybin and dimethyltryptamine (DMT) candidates, a robust intellectual property portfolio, and a multinational operational footprint. The company has recently announced positive topline results from its Phase 2 trial of CYB003, a deuterated psilocybin formulation, in patients with major depressive disorder (MDD). The trial met its primary endpoint of safety and tolerability, and also showed impressive efficacy results, with 79% of patients achieving remission from depression after two doses of CYB003 (12mg).
This is a remarkable achievement, considering that current antidepressants have low remission rates, take weeks to months to work, and cause significant side effects. Cybin’s deuterated psilocybin has the potential to offer a rapid, durable, and well-tolerated treatment option for millions of people suffering from MDD, which is the leading cause of disability worldwide. Cybin plans to initiate a Phase 3 trial of CYB003 in MDD by the end of the first quarter of 2024, pending regulatory approval.
Cybin is also advancing its deuterated DMT programs, which aim to leverage the unique pharmacological properties of DMT, such as its short duration of action and its ability to induce profound mystical experiences. Cybin has two deuterated DMT candidates in development: CYB004, which is being evaluated for generalized anxiety disorder (GAD), and SPL028, which is being developed in collaboration with the University of Miami for treatment-resistant post-traumatic stress disorder (PTSD). Cybin expects to report Phase 1 data for both candidates soon and to initiate a Phase 2 trial of CYB004 in GAD in early 2024.
In addition to its clinical programs, Cybin is also investing in research and development of novel psychedelic molecules, delivery systems, and digital therapeutics. The company has a strong patent portfolio, with 40 granted patents and over 170 pending applications, covering various aspects of its psychedelic platform. Cybin is also building state-of-the-art facilities in Canada and Jamaica to support its preclinical and clinical activities.
We believe that Cybin is well-positioned to capitalize on the growing interest and demand for psychedelic therapies, which have the potential to transform the mental health landscape. The company has a clear vision, a seasoned management team, and a solid financial position. As of September 30th, 2024, Cybin had $76.9 million in cash and cash equivalents, which is expected to fund its operations until mid-2025.
Small Cap Exclusive has identified 3 main catalysts that could drive Cybin’s stock price higher in 2024:
#1 The initiation of the Phase 3 trial of CYB003 in MDD, which could be the first pivotal trial of a psychedelic drug in this indication. PRESS RELEASE
CYBN announced that the United States Patent and Trademark Office (“USPTO”) has granted U.S. patent 11,834,410 in support of its CYB003 program.
The patent, which is expected to provide exclusivity until at least 2041, includes composition of matter claims to pharmaceutical compositions within the Company’s proprietary deuterated psilocybin analog program, CYB003, as well as claims directed toward the therapeutic treatment of major depressive disorder (“MDD”), treatment-resistant depression, and alcohol use disorder.
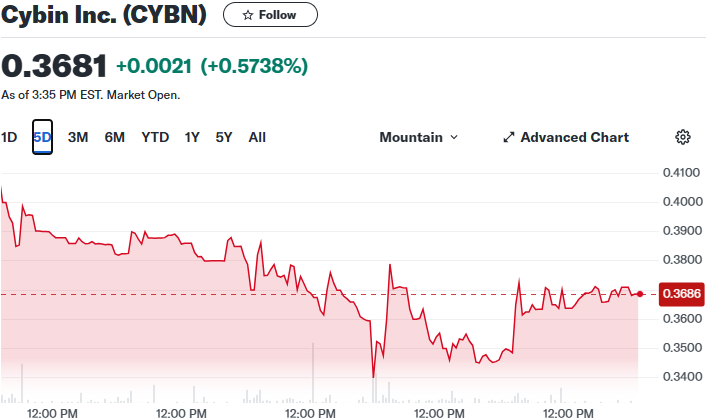
Take a look at the results found from their investors deck on the clinical for CYB003.
How large is the major depressive disorder MDD industry?
According to a report by ReportLinker, the market size of MDD in the seven major markets (the United States, EU5, and Japan) was USD 3,960.6 million in 2020 otherwise known as 3.9B, and the market is estimated to increase at a CAGR of 8.91% for the study period (2018–2030). Another report by Business Research Insights projected that the global major depressive disorder market size was expected to be USD 3985 million in 2021 and will touch USD 6.6B by 2027, at CAGR of 8.9% during the forecast period.

These projections indicate that the MDD industry is a lucrative and promising market for investors who are interested in investing in a clinical stage company and can offer several advantages, such as:
- Access to innovative and cutting-edge therapies that can address unmet medical needs and improve patient outcomes
- Potential for high returns on investment if the products succeed in clinical trials and gain market approval
- Opportunity to contribute to the advancement of science and medicine and make a positive impact on society
Doug Drysdale, Chief Executive Officer of Cybin also contributes to the news of the phase 3 trial by adding, “Securing robust patent protection for our proprietary products is a top priority, and we are pleased to announce this additional U.S. patent supporting our CYB003 program.” “Last week, we shared positive Phase 2 topline safety and efficacy data for CYB003 in MDD. In addition to the rapid, robust and statistically significant reduction in depression symptoms observed three weeks following a single 12 milligram dose of CYB003 compared to placebo, we also saw a sustained improvement at six weeks, as well as impressive response and remission rates of 79% for patients who received two doses. We are extremely pleased with the data and the potential to deliver meaningfully improved treatment options for people with mental health disorders and are eager to progress our CYB003 program through the next regulatory steps.”
#2 The initiation of the Phase 1 trial of CYB004 in GAD, which could be the first clinical trial of a deuterated DMT formulation in this indication. PRESS RELEASE
Cybin nnounced positive safety, pharmacokinetic (“PK”) and pharmacodynamic (“PD”) data from its Phase 1 studies of CYB004 (IV) and SPL028 (IV and IM) in healthy volunteers. CYB004 and SPL028 are proprietary deuterated DMT molecules within the Company’s DMT program in development for the treatment of generalized anxiety disorder.
Results from the Phase 1 studies in the CYB004 and SPL028 programs demonstrated PK and PD profiles with the potential to bridge data across these molecules, and the PK profiles for both molecules demonstrated concentrations in the effective range. Both IV (CYB004 and SPL028) and IM (SPL028) administration routes were safe and well-tolerated, with potential for IM dosing to provide a more convenient dosing method for patients when compared to IV infusion. IM dosing of SPL028 produced robust psychedelic effects lasting a short duration in the majority of subjects, a finding that supports IM administration as a well-tolerated and effective dosing method that is highly scalable.
#3 The announcement of Phase 1 data for CYB004 and SPL028, which could provide further validation of Cybin’s deuterated DMT platform. PRESS RELEASE
CYBN announced positive safety, pharmacokinetic (“PK”) and pharmacodynamic (“PD”) data from its Phase 1 studies of CYB004 (IV) and SPL028 (IV and IM) in healthy volunteers. CYB004 and SPL028 are proprietary deuterated DMT molecules within the Company’s DMT program in development for the treatment of generalized anxiety disorder.
Results from the Phase 1 studies in the CYB004 and SPL028 programs demonstrated PK and PD profiles with the potential to bridge data across these molecules, and the PK profiles for both molecules demonstrated concentrations in the effective range. Both IV (CYB004 and SPL028) and IM (SPL028) administration routes were safe and well-tolerated, with potential for IM dosing to provide a more convenient dosing method for patients when compared to IV infusion. IM dosing of SPL028 produced robust psychedelic effects lasting a short duration in the majority of subjects, a finding that supports IM administration as a well-tolerated and effective dosing method that is highly scalable.
Based on the aforementioned catalysts and the price targets, we are bullish on Cybin’s prospects and recommend it as a stock to watch in 2024.
Speaking of price targets, the average analyst price target for Cybin is $6.00, with a high estimate of $10.00 and a low estimate of $3.00 . This represents a significant upside from the current price of $0.36. (1) We believe that Cybin has the potential to deliver superior returns for investors who are looking for exposure to the psychedelic sector.
Take a look at the screen shot below, it seems like all of Wall Street is as excited about this stock as we are.
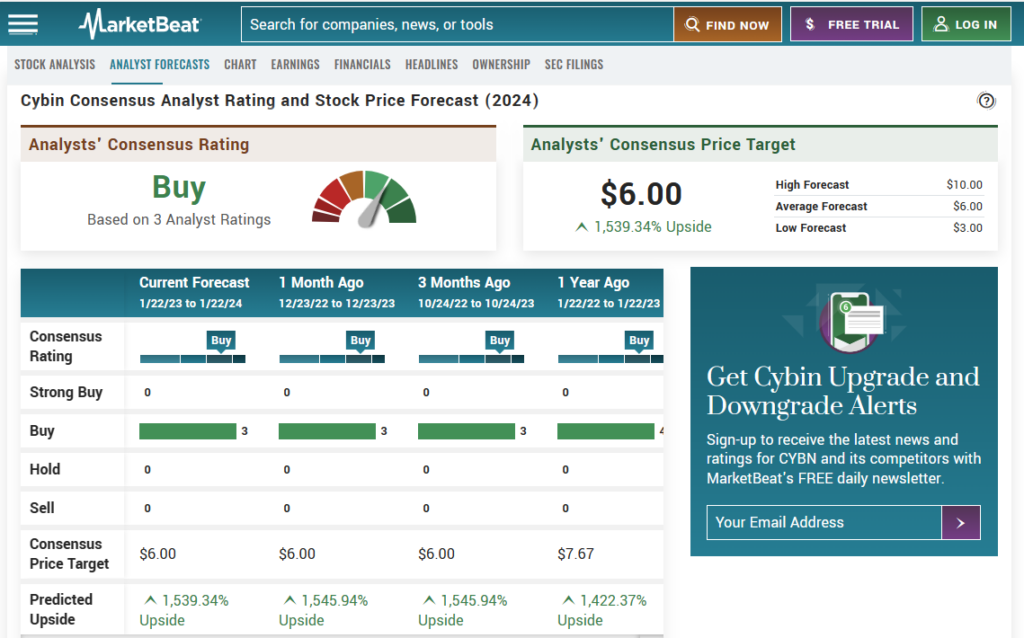
Numerous analysts agree with Small Cap Exclusive that Cybin has the possibility of a 1,500% upside and there is no wonder why these analysts are so excited.
Furthermore, yesterday’s chart looks like it could be ready to move.
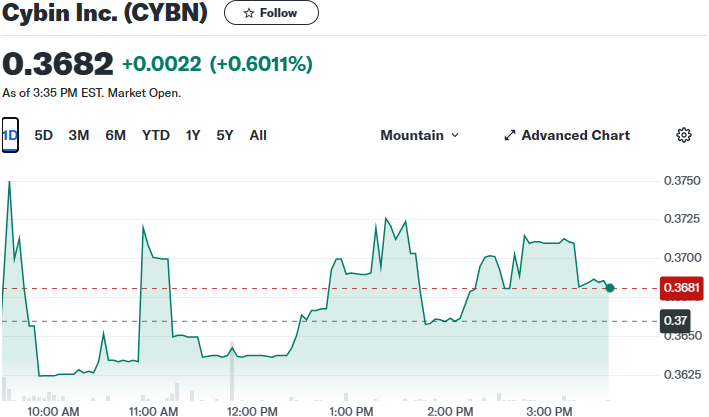
Now that we are excited about the chart, let’s recap the amazing news coming out of this burgeoning anti-depressant juggernaut.
#1 The initiation of the Phase 3 trial of CYB003 in MDD
#2 The initiation of the Phase 1 trial of CYB004 in GAD
#3 The announcement of Phase 1 data for CYB004 and SPL028
When you combine a chart that looks ready for a move, incredible price targets reflecting massive possible future gains and news that is hot as it gets, you may want to turn your attention to Cybin (NASDAQ:CYBN) before it’s too late!
Sincerely,
Small Cap Exclusive Team
Condensed Disclaimer
Small Cap Exclusive is owned and operated by King Tide Media, LLC, which is a US based corporation & has been compensated up to $10,000 from ACN, LLC for profiling CYBN with coverage beginning 1/22/24-1/23/24. We own ZERO shares in CYBN.




